
PEDIATRIC MEDICAL CASE REPORTS


1
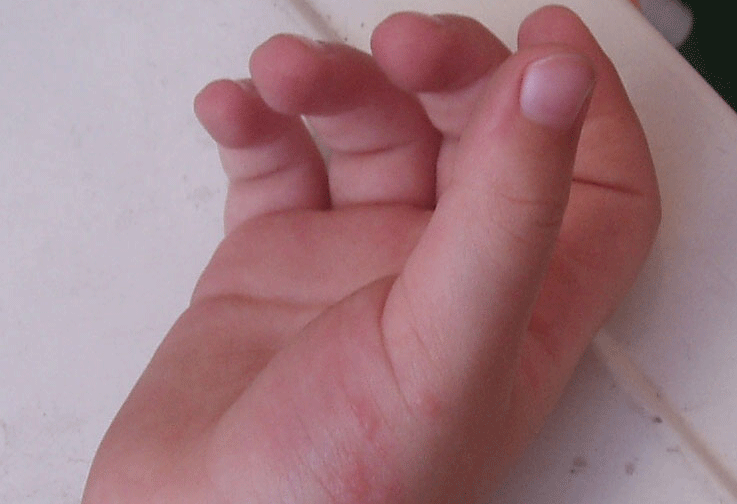
This boy have erythema and pruritus on the thenar of his hand.
He didn't used any medications before the onset of pruritus.
Parents refer that they were in summer vacation to the mountains 3 weeks ago.
His Family Doctor gave to him topical antibiotic ointment and oral antihistamine, but the patient does not feel better, and the symptoms were aggravated.
Which is the Diagnosis and Treatment?
1. Impetigo
2. Scabies
3. Psoriasis
4. Contact dermatitis
Diagnosis is: Scabies
treatment:
Two medications commonly prescribed are permethrin (Elimite, Acticin) and crotamiton (Eurax). Although these medications kill the mites promptly, you may find that the itching doesn't stop entirely for several weeks.
Doctors sometimes prescribe oral medications for people with altered immune systems or for people who don't respond to the prescription lotions and creams.
Because scabies spreads so easily, your doctor may recommend treatment for all family members and other close contacts, even if they show no signs of scabies infestation.
2
An 18-month-old female presents with 2 days of fever and irritability.
Her past medical history is unremarkable.
On physical examination, she is febrile, ill appearing, and has neck stiffness.
The following soft tissue lateral of the neck X-ray is obtained.
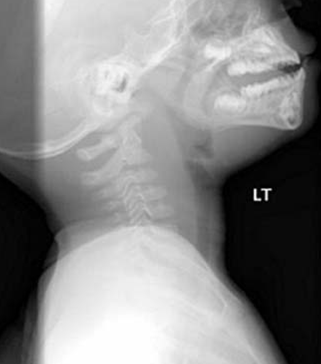
1.
2. What is your diagnosis?
3. What do you expect to see during your physical examination?
4. Give at least 3 differentials, that would make use of radiographic evaluation to differentiate between them.
(1) Answer:
D-Admit for intravenous antibiotics and surgical consultation
(2) Diagnosis: Retropharyngeal abscess
(3) On physical examination, one might see bulging of the posterior pharyngeal wall, although given the age of the involved patients and the likely difficulty of the examination, this may not be obvious.
Involved organisms are most commonly group A Streptococcus, Staphylococcus aureus, and the anaerobes that occupy the oral cavity.
(4) Radiographic evaluation of children with the above complaints mandates differentiation of RPA from other etiologies including epiglottitis, foreign body aspiration, and meningitis. Computerized tomography (CT) scanning with contrast is
frequently used to further characterize the nature of the swelling and is more sensitive in evaluating the difference between cellulitis and true abscess.
Most patients with retropharyngeal abscess are febrile. Some appear toxic and irritable. Cervical lymphadenopathy, usually unilateral, is the most common physical finding in these patients. Patients may have decreased or painful range of motion of their necks or jaws. A neck mass or tenderness may be appreciated. These patients may present with a muffled "hot potato" voice (ie, dysphonia) or with a voice that sounds like a duck quack
Upon inspection of the oral cavity (usually with a tongue blade), the physician may be able to appreciate a mass in the posterior pharyngeal wall. "Tracheal rock sign" elicits pain while gently moving the larynx and trachea from side to side. These patients prefer to lie supine with their necks extended, maximizing their airway patency. Sitting up or flexing their necks worsens their respiratory distress.
**************************
3
A 1 year old infant with sneezing and coughing
A 11-month-old infant has had a runny nose and has been sneezing and coughing for 3 days.
The patient prezented at Pediatric with cough became much worse.
On physical examination, he is in moderate respiratory distress with nasal flaring, hyperexpansion of the chest, and easily audible wheezing without rales.
The diagnosis is:
a. Bronchiolitis
b. Tonsilitis
c. Viral croup
d. Asthma
e. Epiglottitis
f. Diphtheria
And most likely etiology is:
a. Respiratory syncytial virus
b. Staphylococcus aureus
c. Corynebacterium diphtheriae
d. Echovirus
e. Haemophilus influenzae
Correct Answer
the correct answer is:
The diagnosis a. Bronchiolitis
The most likely etiology a. Respiratory syncytial virus
**************************
4
An 11 years old girl complaining of pain in her right shin
An 11-year-old girl with no past medical history presents to the Emergency Department complaining of pain in her right shin for the past month, specifically in the area just under her kneecap.
She was recently on vacation and did a lot of walking, which seems to have worsened the pain.
She also noticed some swelling in the same area for about a week prior to visiting the Emergency Department.
The swelling and pain have both improved somewhat with the use of ibuprofen. The patient and her parents deny any trauma to the area. Additionally, there is no history of fever, numbness, tingling, weakness, or other bone or joint pain.
On physical examination, the patient’s vital signs are within normal limits, with an unremarkable head and neck, chest, and abdominal examination.
In the extremities, the patient has intact sensation to light touch in the L3-S1 distribution. She had 5/5 strength in the bilateral hip flexors, 4/5 strength in the right quadriceps limited by pain over the tibial tubercle, and 5/5 strength in the hamstrings, tibialis anterior, gastrocsoleus, and extensor hallucis longus.
She has brisk distal capillary refill. There is mild soft-tissue swelling (see Image 1) with localized tenderness to palpation over the right tibial tubercle.
There is no erythema overlying the affected area.
A plain film radiograph was obtained (see Image 2).

What is your diagnosis?
a. Osteomyeilitis
b. Osgood-Schlatter disease
c. Osteogenic sarcoma
d. Osteoid osteoma
e. Blount disease
Answer
its a common entity seen in adoloscents especially after heavy atheletic activity
pathology is
Bone growth is faster than soft tissue growth, which may result in muscle tendon tightness across the joint and loss of flexibility.
During periods of rapid growth, stress from contraction of the quadriceps is transmitted through the patellar tendon onto a small portion of the partially developed tibial tuberosity. This may result in a partial avulsion fracture through the ossification center. Eventually, secondary heterotopic bone formation occurs in the tendon near its insertion, producing a visible lump. Approximately 25% of patients have bilateral lesions.
**************************
5
A 40-Day-Old Boy With Facial Swelling
Background
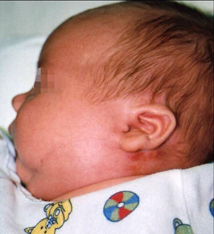
A 40-day-old white male infant with a previously uncomplicated neonatal course presents with a 2-day history of expanding swelling and erythema involving the left angle of the mandible. The infant has been irritable and inconsolable for the 12 hours before presentation. His parents are very distraught. They tried feeding, rocking, and burping the infant, but nothing relieved the crying. According to the parents, the patient had a fever on the day before admission. The infant was a 7.5-lb (3.4-kg), term baby delivered via normal, spontaneous vaginal delivery to a 26-year-old mother who was positive for group B Streptococcus (GBS), rubella-immune, and negative for hepatitis B surface antigen. Rupture of the membranes occurred 10 hours before delivery, with clear fluid. The intrapartum medications included 2 doses of clindamycin for the positive GBS test, which was performed at 35 weeks of gestation. There was no perinatal maternal fever. The Apgar scores were 8 and 9 at 1 and 5 minutes, respectively.
The physical examination shows an irritable infant with a rectal temperature of 101.5°F (38.6°C). In the emergency room, the infant has a heart rate of 120 bpm, a respiratory rate of 48 breaths/min, and a blood pressure of 100/58 mm Hg. A 4-cm area of indurated, nonfluctuant, tender swelling and erythema involving the left subauricular and submandibular facial areas and extending along the line of the jaw to the chin is noted (see Figure 1). The floor of the mouth is soft and the uvula is midline and not enlarged. There is no pharyngeal erythema or exudates. No rhinorrhea is evident. The fontanelle is soft and flat. The lungs are clear to auscultation bilaterally and there are no retractions or nasal flaring. No heart murmur is detected and the heart sounds are normal. The rest of the physical examination is unremarkable.
The peripheral leukocyte count at admission is 10.2 × 103/mm3 (10.2 × 109/L), with 13% bands, 61% neutrophils, 13% lymphocytes, and 3% monocytes. Sodium, chloride, potassium, and bicarbonate are within normal limits for the patient's age. A lumbar puncture is performed and reveals cerebrospinal fluid (CSF) with protein of 67 mg/dL, glucose of 59 mg/dL, leukocytes of 2/mm3, and no red blood cells. Gram stain and agglutination studies are negative. The urine analysis and urine culture results are unremarkable.
Hint: The mother had a positive GBS test at 35 weeks of gestation.
Discussion
Treatment of a culture-positive GBS mother decreases the likelihood of early-onset GBS infection in the infant; however, preventing late-onset GBS infections, such as cellulitis-adenitis, is not as certain. Cellulitis-adenitis syndrome is a disease of great concern because there is a potential for upper airway obstruction and septic shock, which may lead to significant morbidity and mortality. This case exemplifies the importance of including GBS infection within the differential diagnosis of newborn infections.
Group B Streptococci (GBS), or Streptococcus agalactiae, are gram-positive encapsulated diplococci. GBS organisms are facultative anaerobes that, when cultured, form chains or diplococci in liquid media and small white-gray colonies on solid medium. This organism usually produces a narrow zone of B-hemolysis on blood agar, and most strains are resistant to bacitracin. Definitive identification of GBS is through latex agglutination testing for the Lancefield group B carbohydrate antigen. Individual strains of GBS are serologically classified according to their capsular polysaccharides. Nine serotypes of GBS are currently defined and are classified as types Ia, Ib, II, III, IV, V, VI, VII, and VIII. All serotypes have been implicated in causing GBS early-onset disease (occurring at less than 7 days of age); however, serotypes Ia, Ib, II, III, and V are the most common types identified in the United States.Ninety percent of cases of GBS late-onset disease (occurring at 7 days of age or later), including cellulitis-adenitis syndrome, are caused by serotype III. Compared with other serotypes, GBS serotype III is also more readily taken up by brain endothelial cells in vitro, which helps explain the occurrence of GBS serotype III late-onset meningitis.
In the late 1960s, GBS was identified as a major neonatal pathogen. The incidence over the following 2 decades ranged from 1.0 to 5.4 per 1,000 live births in the U.S. In the 1990s, maternal chemoprophylaxis against GBS resulted in a 65% decrease in the presentation of early-onset disease, from 1.7 to 0.6 per 1,000 live births; however, the incidence of late-onset disease remained at 0.4 per 1,000 live births. In 2003, the incidence of early-onset disease had decreased to 0.3 cases per 1,000 live births, equaling the incidence of late-onset disease. Cellulitis-adenitis syndrome, a rare late-onset disease presentation of GBS infection, represents less than 1% of late-onset GBS disease.Since cellulitis-adenitis syndrome is so rare, the incidence of CNS involvement is unknown.
GBS are common organisms in the maternal genitourinary and gastrointestinal tracts, with approximately 30% colonization among pregnant women. Vaginal and rectal colonization are implicated in the vertical transmission of GBS to infants.Without maternal chemoprophylaxis of GBS-colonized women, about 50% of infants will become infected, with 1-2% of those developing invasive disease. Without serotype-specific immunoglobulin G (IgG), the capsular polysaccharide of the GBS organism acts as a virulence factor, preventing activation of the alternative complement pathway and blocking subsequent opsonization and phagocytosis. IgG serum levels higher than 1-2 µg/mL are effective in killing the GBS organism; however, only 10-20% of women have these levels, so most newborns lack serotype-specific antibody protection against GBS.Premature infants have a decreased transplacental transfer of maternal antibodies; therefore, they are at an increased risk for GBS colonization and resultant infection. Other risk factors for colonization include prolonged rupture of membranes (greater than 18 hours), intrapartum fever (temperature greater than 100.4°F [38°C]), maternal GBS bacteremia during pregnancy, a previous infant with GBS disease, absent or low serotype-specific serum antibody, or a high GBS maternal disease burden. Other GBS virulence factors include surface proteins that help adhesion to host cells; C5a peptidase, which inhibits neutrophil recruitment to the site of infection; B-hemolysin, which causes cell injury; and hyaluronidase, which acts as a spreading factor in host tissues.There are 2 proposed possibilities for the pathogenesis of GBS cellulitis-adenitis syndrome. The first involves mucus membrane colonization with GBS, followed by bacteremia and seeding of the soft tissues. The second is ipsilateral otitis media, with subsequent lymphatic spread and bacteremia.
Other possibilities that should be considered in the differential diagnosis of GBS sepsis include neonatal respiratory distress syndrome and transient tachypnea of the newborn. In cases with associated meningitis, other common organisms responsible for infant meningitis, such as Escherichia coli and Listeria monocytogenes, must be considered and treated empirically.
The typical presentation is divided into early-onset and late-onset GBS infection. Early-onset GBS infection is limited to the first 5 days of an infant's life; most often, the infection appears within the first 24 hours. The infants most at risk for early-onset GBS are those with maternal GBS colonization, low birth weight, and delivery complications, including premature labor, rupture of the membranes more than 24 hours before birth, and maternal fever. The most typical symptom is respiratory distress, which may be followed by apnea and cardiovascular collapse. The child may have sepsis in association with pulmonary infiltrates. Thirty percent of infants will also have meningitis. In such cases, GBS infection is often confused with E. coli infection. Acute respiratory distress is the most predictable first sign of infection and is present in 84% of cases of GBS. A low Apgar score at 1 minute, a low absolute neutrophil count, a history of prolonged rupture of membranes, and the presence of GBS in the gastric aspirate are some of the criteria used to differentiate GBS from neonatal respiratory distress syndrome.
Late-onset presentation occurs later than 7 days after birth, with many cases presenting around 24 days of age. This form of GBS typically manifests as meningitis (seen in up to 80% of late-onset cases). It may also present as cellulitis-adenitis, which is most common in male infants between 3 and 7 weeks of age. The symptoms include acute suppurative cervical lymphadenitis, fever, otitis media, bacteremia, and poor feeding. There are many serious complications associated with late-onset GBS; these include cortical blindness, central diabetes insipidus, deafness, poor thermal regulation, and subdural effusions. Infants presenting with meningitis may develop hypotension, seizures, coma, and leukopenia, which are poor prognostic factors. Some rare presentations of late-onset GBS infections include asymptomatic bacteremia, otitis media, cellulitis, conjunctivitis, abscess, endocarditis, pericarditis, and osteomyelitis. Bacteremia is most common at 2-4 weeks of age, and it can be diagnosed by blood culture. In one study, a series of 9 infants with GBS osteomyelitis showed a propensity for localizing infection to 1 bone (most often, at the proximal humerus, and also at the proximal and distal femur).
In cases of cellulitis-adenitis, ultrasonography may be used to diagnose suppuration of the lymph nodes. In addition, a fine-needle biopsy, blood cultures, or aspiration of bone or joints to check for osteomyelitis may be indicated. Cultures should show beta-hemolytic bacteria that are resistant to bacitracin. Counterimmunoelectrophoresis and latex particle agglutination (LPA) are sensitive and specific tests for GBS infection. A lumbar puncture with signs of bacterial infection consistent with GBS is diagnostic for meningitis. Although LPA is useful for confirming the diagnosis, it is not routinely available.
Initial empiric treatment of neonatal GBS infection requires antimicrobial coverage consisting of ampicillin and an aminoglycoside or cefotaxime. For confirmed GBS with documented sensitivities, penicillin G is the antibiotic of choice. The dosage depends on the patient's age. Infants 7 days of age and younger require 250,000 to 450,000 U/kg/day of IV penicillin G divided into 3 daily doses. Infants older than 7 days of age require 450,000 to 500,000 U/kg/day of IV penicillin G divided into 4-6 doses daily.The location and extent of infection will dictate the duration of treatment. Bacteremia without a focus requires at least 10 days of treatment, meningitis requires 2-3 weeks, while ventriculitis and osteomyelitis require 4 weeks of treatment.The necessity of a second lumbar puncture after 24-48 hours of therapy to document CSF sterility remains controversial. Additionally, nafcillin is active against GBS in vitro, but it is not effective for GBS meningitis because it does not reach bactericidal CSF concentrations.
GBS infection is a serious condition. The fatality rate for early-onset neonatal GBS disease is 4.7%, and it is 2.8% for late-onset disease. There may be long-term effects of GBS infections. Up to 30% of infants who survive GBS meningitis have serious long-term neurologic sequelae. Long-term complications can include developmental delays, microcephaly, seizure disorders, blindness, deafness, and quadriplegia.
Routine screening for GBS colonization is done with vaginal and rectal GBS cultures at 35-37 weeks gestation for all pregnant women. Women with a prior infant with invasive GBS disease or GBS bacteriuria during a current pregnancy are exempt from routine screening because they will be treated regardless of the culture results. For women found to be culture-positive with an unknown GBS status, delivering at less than 37 weeks gestation, with greater than 18 hours of ruptured of membranes before delivery, or an intrapartum temperature greater than 100.4°F (38°C), treatment is administered during labor. Patients undergoing planned cesarean section without labor or membrane rupture do not require treatment, regardless of the results of the culture.
Prevention of GBS infection in infants relies on the intrapartum administration of penicillin G to mothers, given at an initial dose of 5,000,000 U, then 2,500,000 U every 4 hours until delivery; alternatively, ampicillin can be administered. There are no reports of resistance to penicillin G, but GBS resistance to erythromycin and clindamycin has been reported at 15-30% and 10-20% of cases, respectively Cefazolin is an alternative for those women allergic to penicillin. Vancomycin is reserved for patients with a high anaphylactic risk and those for whom susceptibility to erythromycin and clindamycin has not been performed or has been found resistant. Administration of antibiotics prior to the onset of labor has shown to be ineffective for preventing neonatal GBS disease. Unfortunately, while the treatment of GBS has shown a decrease in the incidence of early-onset GBS infections, it has not had an effect on late-onset GBS infections (such as cellulitis-adenitis).
Clinical trials have studied the possibility of a GBS vaccine, using the capsular polysaccharide to provide transplacental passage of GBS antibodies to the infant. The vaccine would eliminate the need for routine cultures during pregnancy and antibiotic prophylaxis, and would likely have an effect on both early-onset and late-onset GBS disease.Currently, no vaccines are routinely used in the United States for the prevention of GBS disease.
GBS cellulitis-adenitis was part of the differential diagnosis upon initial outpatient presentation of the patient in this case. He presented with fever, irritability, and swelling under the left ear. A full septic evaluation was initiated, including a complete blood cell count (CBC) with differential, electrolytes, urine analysis, urine culture, and CSF studies. The patient was also started on empiric antibiotic treatment with ampicillin and cefotaxime at meningitic doses. Despite the mother being treated with 2 doses of clindamycin prior to vaginal delivery, the infant's blood culture was positive for GBS type III infection. The CSF culture was negative. The treatment was then modified to ceftriaxone for a total of 14 days. The patient had a complete recovery.
6
An 8-Day-Old Boy With Tachypnea and Difficulty Feeding

Background
An 8-day-old boy is brought into the emergency department (ED) by his parents for a chief complaint of rapid breathing. During the past several hours, the patient's mother noted an increased respiratory rate and decreased feeding ability. The child had previously fed for 10 minutes at each breast every 2 hours, but he is now crying and unable to latch on for more than a few seconds at each breast. Both parents state that they think the patient looks pale. Up until the day of presentation, the patient had been doing well, with no prior episodes of difficulty breathing. He was born full-term via normal spontaneous vaginal delivery, without complications during the pregnancy or delivery. He has had no sick contacts and has not been in day care. He has not had a fever, cough, or runny nose. His urine output has been normal, and he has had 2-3 nonbloody bowel movements daily. Other than spitting up a small amount after feedings, there have not been any episodes of vomiting. The parents state that he seems increasingly fussy. There have been no episodes of apnea, cyanosis, choking, or gagging, as well as no discernible changes in muscle tone.
On physical examination, his vital signs are significant for a respiratory rate of 70 breaths/min and a heart rate of 296 bpm. He is afebrile, with a rectal temperature of 98.2°F (36.8°C). His blood pressure is 72/40 mm Hg. His oxygen saturation, measured by pulse oximetry, is 98% while breathing room air. Despite the abnormal vital signs, the baby does not appear distressed or even uncomfortable. He is moving all extremities, and he opens his eyes and looks around. His oropharynx is clear, with no visible foreign bodies. There is no rhinorrhea or nasal congestion. His lung sounds are clear, and despite the significant tachypnea, no retractions, grunting, or nasal flaring are present. On auscultation of the heart, rapid heart sounds are noted, and as a result of the tachycardia, an assessment for murmurs is not possible. His distal extremities are pink and have a normal capillary refill.
While placing an intravenous line and connecting the child to a monitor, an electrocardiogram (EKG) is obtained.
Hint: Consider the morphology of the QRS complexes (narrow vs wide) and the timing of the complexes (regular vs irregular).
Discussion
This patient was diagnosed with supraventricular tachycardia (SVT), which is defined as a regular, rapid rhythm that requires only atrial or atrioventricular tissue for its initiation and maintenance. Paroxysmal SVT (PSVT) is the most common dysrhythmia in children. It has an international prevalence of about 2 cases per 1,000 people. PSVT tends to manifest in infancy and early childhood.
The presentation of PSVT is quite variable in children, ranging from an incidental finding in an asymptomatic patient to fulminant cardiogenic shock. Infants present with caretakers complaining of rapid breathing, poor feeding, sweating with feeding, pallor, lethargy, and excessive crying. Older children may complain of chest pain, shortness of breath, and palpitations. The physical examination is likewise variable, depending upon the child's age and heart rate and on the duration of the episode (which can last from seconds to days). For infants, the examination is remarkable for a regular tachycardia. Normal resting heart rates for neonates can be up to 160 beats per minute. The upper limit of the normal heart rate decreases with age until late adolescence, when children's heart rates are similar to those of adults. Heart rates ranging beyond the normal upper limits, particularly in excess of 240, should be highly suspicious for SVT. In addition to tachycardia, infants may have physical findings of pallor, irritability, lethargy, tachypnea, weight loss (or failure to gain), poor perfusion, weak pulses/hypotension, hepatomegaly, and, sometimes, cardiogenic shock. A pounding sensation in the neck may be caused by cannon A waves, which occur when the atrium contracts at the same time as the ventricle. Older children typically have benign examinations (except for the findings of tachycardia and tachypnea).
There are 3 types of SVT: (1) atrial tachycardia (ectopic, or nonreciprocating, atrial tachycardia), (2) atrioventricular nodal reentrant tachycardia (AVNRT), and (3) atrioventricular reentrant (or reciprocating) tachycardia (AVRT). In the United States, reentrant tachycardias are the most common cause of PSVT in the pediatric population, with AVRT being more common than AVNRT. AVRT consists of 2 or more functionally (and, usually, anatomically) distinct pathways between the atria and ventricles. The first pathway is usually the atrioventricular (AV) node. The second is an accessory pathway that may be an anatomically separate bypass tract between the atrium and ventricle (such as the bundle of Kent). Wolff-Parkinson-White (WPW) syndrome preexcitation is a good example of SVT caused by an anatomically separate bypass tract. Each pathway has different electrophysiologic characteristics; one pathway is fast (a short conduction time and a long refractory period), while the other is slow (a longer conduction time and a shorter refractory period). In AVNRT, both pathways exist in the AV node itself. In AVNRT, for example, while the child is in regular sinus, a premature atrial beat may block in the fast pathway (because of its longer refractory period). Since the fast pathway is blocked, the signal conducts down the slow pathway. Then, when the impulse reaches the insertion of the fast pathway, which has now recovered after its refractory period, the impulse conducts in a retrograde fashion through the fast pathway. This results in a circuit loop tachycardia, with an impulse moving in a loop down the slow pathway and up the fast one.
Other causes of PSVT include sympathomimetic stimulation (such as medications for upper respiratory infection), structural defects, and atrial ectopy. About half of all SVT cases occur without underlying heart disease; these cases are termed idiopathic. Idiopathic SVT is more common in younger patients than in older children. WPW syndrome preexcitation accounts for 10-20% of cases. Congenital heart defects may also predispose children to SVT. Children who have undergone cardiac surgery are also more prone to developing this arrhythmia.
The diagnosis of PSVT is made based on patient history, physical examination findings, and EKG findings. The EKG findings include an excessively rapid (usually between 200 and 280 bpm) regular tachycardia, often without discernible P-waves preceding the QRS complexes. P-waves, when visible in PSVT, may have an abnormal axis and may be seen within or following the QRS complexes. In most cases, the tachycardia will be narrow complex; however, wide-complex PSVT is possible if aberrancy is present or if the AVNR conduction occurs in an antidromic fashion with WPW syndrome. It is important to distinguish PSVT from sinus tachycardia, which is the most common tachycardia in children. Sinus tachycardia and PSVT have dramatically different treatments. Laboratory evaluation will depend on the clinical scenario, but practitioners should consider checking electrolytes, thyroid function, and hemoglobin level.
Emergency management of children with PSVT should start with a focus on first assessing the airway for potential compromise or obstruction, then evaluating breathing and ventilation, and assessing the circulatory status of the patient (ie, the "ABCs"). When evaluating the circulatory status, unstable patients should undergo immediate synchronized cardioversion with 0.5 J/kg. The power should be increased as needed to 2 J/kg. If the cardioversion fails, overdrive pacing is another option. For stable patients, vagal maneuvers can be attempted first. Infants possess a diving reflex, in which vagal tone will increase in response to a cold stimulus (eg, ice) on the face. In older children, unilateral carotid massage, eyeball pressure, or even a headstand is more likely to cause conversion. When these maneuvers are not typically successful in converting the rhythm, medication is usually required. Adenosine, given at a dose of 0.1 mg/kg rapid intravenous push, is the first-line agent. This may be repeated in doses of 0.3 mg/kg, as needed. Amiodarone and procainamide are also safe in children with SVTs refractory to adenosine. In general, adenosine and digoxin should be avoided in situations where there is a suspicion of WPW syndrome, as they may potentiate conduction through the accessory pathway, resulting in increased ventricular rates and possible degeneration to ventricular fibrillation. Children without WPW syndrome preexcitation can be maintained on chronic oral propranolol or verapamil (in children older than 5 years), if needed after the acute illness. These agents should be used in consultation with a pediatric cardiologist.
Disposition of children with PSVT depends on the clinical circumstance. A child in shock or with concerning comorbidities should be admitted to a pediatric intensive care unit (ICU). Asymptomatic children without frequent recurrence may be able to follow up with a cardiologist, without initiating medications. For younger infants, the admission threshold should be lower.
The patient in this case failed cardioversion with vagal maneuvers but converted after two boluses of adenosine, which is consistent with a reentrant circuit involving the AV node. The patient was admitted to a monitored pediatric unit and evaluated by pediatric cardiology. The patient was initially discharged on digoxin; however, after further follow-up, the medication was discontinued, because the child's cardiologist came to suspect WPW syndrome as the likely etiology for the PSVT.
7
Pneumonia in a 3-Year-Old Boy After Falling Into a Well

Background
A 3-year-old boy is presented to the emergency department (ED) of a regional hospital in northeast Thailand 4 days after falling into a well. He was found by his brother, who went looking for him after he could no longer hear the sound of the boy playing. The boy is not able to swim. Although the patient's relatives estimate that he was in the water for no longer than 5 minutes, they believe that he may have aspirated a significant amount of water. He did not lose consciousness and appeared to recover completely within minutes of being rescued. Three days later, however, the boy developed a fever and cough, and he was noted to be more drowsy than usual; these symptoms prompted his family to bring him to the ED. The child has never been to a hospital before, is normally fit and well, and is on no regular medications. The patient comes from a family of rice farmers and often plays in the paddy fields near his home. Both parents and siblings are well-appearing, and there is no family history of diabetes, thalassemia, or renal disease.
On physical examination, the boy is noted to be drowsy, but he is obeying instructions from his mother. His temperature is 102ºF (38.9ºC), his respiratory rate is 36 breaths/min, and his pulse rate is 132 bpm and regular. The patient's blood pressure is not recorded, and a pulse oximetry reading is not available in this setting (because of a lack of equipment). He is not clinically cyanotic or jaundiced, and there are no peripheral signs of chronic disease. The patient is noted to have a moderately increased work of breathing, with use of the accessory muscles of respiration; in addition, widespread fine crackles are audible over the chest bilaterally. The heart sounds are normal, with no rubs or murmurs. There is no palpable lymphadenopathy, no visible skin lesions, and no palpable organomegaly. The abdominal examination is unremarkable.
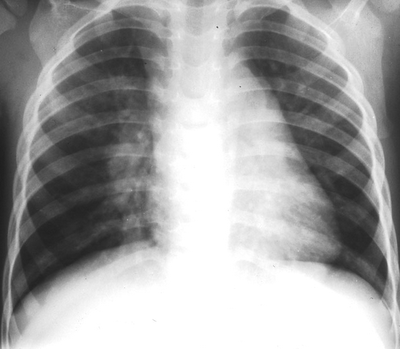
The patient is placed on supplemental oxygen, which leads to some improvement in his respiratory status. An intravenous (IV) line is placed and initial laboratory investigations are performed. The results of the investigations show a blood count with a hemoglobin of 8.7 g/dL (87 g/L), a mean corpuscular volume of 60.1 µm3 (60.1 fL), a platelet count of 196 × 103/µL (196 × 109/L), and a total white blood cell (WBC) count of 4.51 × 103/μL (4.51 × 109/L; 53% neutrophils [0.53], 32% lymphocytes [0.32], and 12% monocytes [0.12]). On a complete chemistry panel, he is noted to have a sodium of 134 mEq/L (134 mmol/L), a potassium of 3.0 mEq/L (3.0 mmol/L), a chloride of 98 mEq/L (98 mmol/L), a bicarbonate of 24 mEq/L (24 mmol/L), a blood urea nitrogen (BUN) of 8 mg/dL (2.9 mmol/L), and a creatinine of 0.3 mg/dL (26.5 µmol/L). No arterial blood gas or glucose measurements are available. The admission chest radiograph (Figure 1) demonstrates bilateral diffuse infiltrates. The decision is made to initiate broad spectrum antibiotic therapy, with intravenous ceftriaxone and metronidazole, and the patient is admitted to the hospital.
Hint: Note the contact with surface water and that the location is in northeast Thailand.


The plain chest radiograph taken on admission (Figure 1) showed diffuse bilateral infiltrates consistent with bilateral pneumonia. The patient was initially treated with broad spectrum intravenous antibiotics, including ceftriaxone and metronidazole. Unfortunately, after 3 days of therapy, the patient continued to have spiking fevers, and his condition was determined to have failed to respond the treatment. The patient was transferred to the pediatric service of the hospital and empirical ceftazidime was initiated. Based on the geographic location of the case, the clinical history of aspiration of well water, and the nonresponse to standard antibiotic therapy for pneumonia, the diagnosis of melioidosis was suspected by the consulting pediatric infectious disease specialist.
During the rainy season, Burkholderia pseudomallei (formerly Pseudomonas pseudomallei) is the organism most commonly isolated in cases of community-acquired bacteremia in northeast Thailand and northern Australia.It is an aerobic, motile, gram-negative, saprophytic bacterium commonly found in soil and surface water in Southeast Asia and northern Australia. B pseudomallei infection is known as "melioidosis" (from the Greek words melis, which means "distemper of asses," and eidos, which means "the likeness of"). The disease is probably endemic to the entire worldwide land area between latitudes 20° N and 20° S; sporadic cases have been reported in Central and South America, the Caribbean, and eastern and western Africa.
The signs and symptoms of melioidosis are protean. A definitive diagnosis of melioidosis can only be made by identifying the causative organism from a clinical specimen. Given the lack of microbiologic facilities in many parts of the tropics, the prevalence of melioidosis is almost certainly underestimated. In the United Kingdom, there were 5 cases of melioidosis imported from Bangladesh between 1988 and 1998; however, Bangladesh only reported its first case in 1998.Likewise, 2 cases of melioidosis imported from Honduras were reported in 2005 in the United States, yet Honduras itself has never reported a case of melioidosis. In Thailand, the first case of melioidosis was reported in 1955, but the disease remained largely unrecognized until the availability of routine microbiologic testing in the mid-to-late 1970s.
In endemic areas, melioidosis most commonly presents as fulminant sepsis, and the duration of symptoms experienced before presentation is commonly brief. There are usually (but not invariably) lesions in the lungs (presenting as consolidations or as lung abscesses), liver, spleen, kidneys, skin, and joints.The mortality of melioidosis is high (20-40%), despite adequate and appropriate treatment. Melioidosis also has a less-common indolent form, which presents after an extended period of incubation and may be difficult to differentiate from tuberculosis. This is the form most commonly seen in nonendemic areas (eg, the United States and Western Europe), where the majority of cases are imported.
A history of contact with soil or surface water is almost always part of the history; specific occupations, such a rice farming and gardening, are important risk factors for the development of disease. The most common route of infection is presumed to be translocation via minor abrasions in the skin, but inhalation is probably an important route as well (as occurred dramatically in this case, with aspiration of contaminated well water; this has also been described in cases of extreme weather events).Around two thirds of all adult patients with melioidosis have diabetes mellitus as a risk factor and, in endemic areas, melioidosis may be the first presentation of diabetes. As a result, all adult survivors of melioidosis must be tested for diabetes following recovery. Other risk factors for melioidosis include immunosuppression (corticosteroids, methotrexate, chemotherapy for cancer), renal disease (chronic renal impairment or renal calculi), thalassemia major, excessive alcohol use, cystic fibrosis, and malignancy.A study of over 500 patients with melioidosis found no association with human immunodeficiency virus (HIV) infection.Melioidosis in children differs in a number of ways from that seen in adults. In children, the disease is usually a localized abscess, mortality is low, relapse is an uncommon occurrence, and, often, contact with soil and water are the only risk factors.Around one third of all pediatric disease presents as parotitis.
B pseudomallei is not a normal commensal of humans, and a carrier state is exceedingly rare. Identification of B pseudomallei in any clinical specimen should, therefore, be considered diagnostic of melioidosis. A minimum of blood, throat swab, respiratory secretions (eg, sputum, tracheal aspirate, bronchoalveolar lavage), and urine should be collected from every patient in whom the disease is suspected.Additionally, pus from any abscesses or collections and surface swabs from any wounds should be obtained, as should specimens from any other site as clinically indicated. Nasogastric aspirates are useful in young children, who tend to swallow their sputum instead of expectorating it. Throat swabs are a valuable diagnostic tool and are often positive, even in cases where there is no evidence of oropharyngeal or respiratory involvement.
B pseudomallei should be handled in biosafety level 3 conditions because of the risk of laboratory-acquired infection.The specimens listed above would normally only be handled in biosafety level 2 conditions; however, the appearance of melioidosis in the differential diagnosis should prompt the clinician to warn the microbiology laboratory before any specimens are sent.
The classic textbook appearance of an intracellular bipolar-staining rod ("safety pin" appearance) is uncommon, not sensitive, and not dependable for diagnosing B pseudomallei. B pseudomallei is not usually fastidious in its nutritional requirements and will grow in a wide variety of media. Early liaison with the microbiology laboratory permits the use of techniques that maximize the chances of identifying the organism. For blood cultures, lysis centrifugation improves the time to identification, but it requires that the microbiology laboratory be contacted before blood is collected because special specimen containers (eg, isolator blood culture tubes) may be required. Specimens taken from nonsterile sites (eg, sputum and throat swabs) are prone to overgrowth by commensal bacteria, thereby obscuring the presence of B pseudomallei. The use of selective media maximizes the chances of isolating B pseudomallei from these specimens. In endemic areas, Ashdown agar (which contains crystal violet and gentamicin as selective agents) and SBCT (tryptic soy broth with crystal violet and colistin) are routinely used for this purpose. These media are cheap, easy to manufacture, and the ingredients are readily available. Commercially available Burkholderia cepacia selective medium is an acceptable substitute for microbiology laboratories that no longer make their own media.
The colonial morphology of B pseudomallei (classically described as looking like cornflower heads; see Figure 2) is exceedingly variable and may look like environmental contaminants (other environmental species, eg, Pseudomonas and Burkholderia, may produce colonies that are similar in appearance). Colonies of B pseudomallei may be erroneously reported by the laboratory as being of no clinical significance, unless the laboratory is specifically warned that melioidosis is suspected. This is unlikely to occur in cases where there is a heavy pure growth of the organism from a sterile site (eg, blood or an intraoperative specimen), but it is possible when growth is meager and the specimen is from a nonsterile site (eg, sputum or a throat swab). Specifically warning the laboratory that melioidosis is suspected is critical, as the presence of even a single colony may clinch the diagnosis. Even if the isolate is recognized as significant, identification of the organism is not straightforward because some commercial systems may erroneously identify the organism as other Burkholderia species, Chromobacterium violaceum, or Pseudomonas species; a 16S polymerase chain reaction (PCR) may be required to properly identify the organism.In the United States, help with identification is available from the Centers for Disease Control and Prevention (CDC); in the United Kingdom, help is available from the Health Protection Agency.
The organism is intrinsically resistant to a wide range of commonly prescribed empirical antimicrobials for aspiration pneumonia (eg, ampicillin, ceftriaxone, metronidazole, moxifloxacin, clindamycin), and identification by culture often takes a minimum of 48 hours. A high level of clinical suspicion is required if appropriate therapy is to be initiated in a timely fashion. Although serologic methods exist for the diagnosis of melioidosis, they are of limited value in endemic areas; serologic methods are more useful for the diagnosis of melioidosis in visitors from nonendemic areas,but seronegativity does not exclude a diagnosis of melioidosis, and culture still represents the gold standard for diagnosis.
The treatment of choice is parenteral ceftazidime (120 mg/kg/d in 3 divided doses; maximum dose, 2g tid); meropenem and imipenem are alternative treatments. Ceftazidime resistance in B pseudomallei is rare, and resistance to carbapenems has not been described. Parenteral treatment should be administered for a minimum of 10 days, and it should be continued until there is a clear clinical improvement (eg, temperature <99.5ºF [<37.5ºC] for at least 48 hours). Patients will have a slow clinical response to treatment. The median fever clearance time is 9 days, despite adequate therapy.Unlike most other bacterial diseases, failure to respond after 48 hours of appropriate treatment is not an indication to revise antibiotic therapy or to consider alternative diagnoses. Although resistance can develop during the course of treatment, this is uncommon; it is, however, prudent to take repeat cultures from any patient who fails to defervesce after 7 days of therapy. Adequate supportive treatment is essential, including fluid resuscitation, artificial ventilation, tight glycemic control, and renal replacement therapy. Adjunctive treatment with granulocyte colony-stimulating factor (G-CSF) has been shown to be ineffective. The use of adjunctive low-dose steroids or activated protein C (drotrecogin alfa) in melioidosis has not been evaluated in randomized controlled trials.
Relapse and reinfection are common; therefore, patients require 20 weeks of oral eradication therapy after completing parenteral treatment. Adults require trimethoprim-sulfamethoxazole (16/80 mg/kg/d in 2 divided doses; maximum dose, 320/1600 mg bid) and doxycycline (4 mg/kg/d in 2 divided doses; maximum dose, 100 mg bid).Children under the age of 8 years and pregnant women (for whom doxycycline is relatively contraindicated) should be given amoxicillin-clavulanate (180/45 mg/kg/d in 3 divided doses).Amoxicillin-clavulanate is also an option for adults unable to tolerate trimethoprim-sulfamethoxazole or doxycycline. Thirteen percent of patients will have a relapse or reinfection in the 10 years following their primary infection; for this reason, patients with B pseudomallei in Thailand are followed up for the remainder of their lives. Patients who have no systemic symptoms and only a single localized abscess that has been completely drained may be treated with oral eradication therapy alone.
In this case, a nasogastric aspirate taken on admission returned culture-positive for B pseudomallei. The patient was continued on intravenous ceftazidime by the pediatric service upon the recommendation of the pediatric infectious disease specialist. He was noted to have spiking fevers of up to 102.2°F (39ºC) until hospital day 5, at which time he defervesced. He was ultimately administered intravenous ceftazidime for a total of 21 days during the hospitalization. The patient made a good recovery and was discharged to home on oral amoxicillin-clavulanate (2:1) syrup 125mg tid for a 5-month follow-up course. He was doing well when seen at follow-up 1 month later.
8
A 3-Year-Old Boy With Intense Itching

Background
A 3-year-old boy presents to his pediatrician with a 1-week history of worsening erythema and pruritus on the thenar aspect of his right hand. He has not used any oral or topical medications before the onset of pruritus. His parents deny any history of allergies, concurrent fever, or other illness. His vaccination schedule is up to date. The patient's recent social history is significant for a summer vacation to the mountains 3 weeks before his parents noticed the skin rash.
The physical examination reveals a healthy-looking boy in no distress. He is in the 50th percentile for weight and 75th percentile for height. His oral temperature is 98.6°F (37.0°C). His pulse rate is 85 bpm, with a regular rhythm. His blood pressure is 110/60 mm Hg. The examination of his head and neck is normal. His lungs are clear to auscultation, with a normal respiratory effort. The patient's S1 and S2 heart sounds are normal, and no cardiac murmurs are noted. His abdomen is soft and nontender, with no organomegaly present. His peripheral arterial pulses in all extremities are normal. Examination of the skin of the right hand reveals a few grouped, blanchable, erythematous papules on the thenar surface of the patient's right hand. Faint excoriations are also noted in the same anatomic location. The child withdraws his hand when the lesions are palpated. The remainder of the total cutaneous examination is unremarkable.
The patient is prescribed a topical antibiotic ointment to prevent infection and an oral antihistamine to decrease the pruritus; however, 1 week later, the patient returns to the clinic with an increased number of lesions and persistent symptoms. Upon further questioning on this repeat visit, the patient's parents note that they had stayed in multiple hotels during their summer vacation. The parents deny knowledge of any skin lesions on any of the other family members who had shared the vacation time with the patient.
A picture of the patient's right hand is shown (Figure 1).
Look closely at the skin 'preceding' the lesions in this child.
A skin scraping was performed and examined under microscopy. The scraping demonstrated mites, ova, and scybala (fecal debris) consistent with a diagnosis of scabies. Scabies is a parasitic infestation of the skin caused by the arthropod Sarcoptes scabiei var. hominis, a very small parasite measuring 0.3 to 0.9 mm in size. Variants of the scabies mite that affect other animals (such as dogs, cats, pigs, etc.) are unable to reproduce in humans, but they can cause a transient dermatitis. Scabies was well known to the Greeks and Romans. The word scabies is derived from the Latin word "scabere," which means to scratch. The Italian biologist Giovanni Cosimo Bonomo described the mite and linked it to the disease in the 17th century; however, it wasn't until the 19th century that the skeptical scientific community finally accepted that scabies was caused by the mite Sarcoptes scabiei.
In the 21st century, scabies continues to be a worldwide health problem. Its prevalence is higher in developing countries, where overcrowding and poverty lead to an increase in transmission. Scabies is transmitted by prolonged skin-to-skin contact with an infected person. Children (especially those of poor or underserved communities), sexually active individuals, and institutionalized patients are all at increased risk for acquiring the disease, because these groups have an increased chance of human contact. Scabies is also considered a sexually transmitted disease. It is infrequently acquired through the use of infested clothes, bed linen, and towels. A scabies infestation occurs when an impregnated female parasite enters the skin, tunnels into the stratum corneum, and excavates burrows to live and deposit its eggs. The larvae hatch and begin to move 3-10 days later. The female mite can sometimes be observed at the end of the burrow. Male mites usually roam on the surface of the skin, but female mites may also surface occasionally (especially at night), at which time they can be washed or scratched off.
Intense pruritus is a classic symptom of scabies, and it is usually more severe at night. Symptoms are caused by a delayed type IV hypersensitivity reaction to the mites, ova, and scybala (feces) under the skin. The symptoms occur 4-6 weeks after the initial infection. Antibodies may form against the parasite causing this reaction; therefore, previously sensitized patients may have symptoms within hours of reinfection.
Scabies can mimic numerous skin conditions, such as insect bites, atopic and contact dermatitis, and herpetic dermatitis (among others). Not uncommonly, scabies is misdiagnosed as one of these other skin conditions, allowing transmission to continue to occur until the correct diagnosis is established. Scabies can be diagnosed by visualizing the burrows or "tracks" left by the female mite as she moves through the stratus corneum of the skin. Initially, these burrows may appear as short, zigzagging, and hardly noticeable scratchlike marks with a slightly shiny hue, and they may be connected to an erythematous papule. This papule harbors the female mite and feces. As a result of the intense pruritus associated with scabies, the burrows may be difficult to differentiate from the scratch marks produced by the patient. Adult patients may show lesions between their fingers or on their wrists, axillae, areolae, elbows, knees, genitals, and buttocks. Alternately, children may have any part of their skin affected. The diagnosis can be confirmed by finding the mite, ova, or fecal debris of a lesion scraping under microscopy. The skin scraping is obtained by scraping infested primary lesions, such as vesicles or burrows, and placing the scraping on a glass slide; multiple scrapings may be necessary to positively make the diagnosis. In rare cases, skin biopsies may be obtained to both positively identify the mite or characteristic histopathology and to rule out other dermatoses.
Related to scratching, patients with scabies may also become superinfected with Streptococcus pyogenes and Staphylococcus aureus. The complication of superinfection can lead to specific infections, such as impetigo, cellulitis, acute poststreptococcal glomerulonephritis, rheumatic fever, and even sepsis. Immunosuppressed patients may present with a severe form of scabies known as crusted scabies or Norwegian scabies. This is a widespread hyperkeratotic rash, with thick scaling and crusting and, at times, minimal to no pruritus. During this type of infection, up to millions of mites may survive for up to 1 week. In nonimmunocompromised hosts, an average of 5-15 mites cause infestation and survive for only 2-3 days. Crusted scabies appears to be the result of a high number of infiltrating CD8+ T lymphocytes in the dermis, with minimal helper T lymphocytes (CD4+), which is the opposite to that of a normal immune reaction to scabies. This detail may account for the body's inability to mount an appropriate immune response against the scabies mites.
The cornerstone of medical treatment for scabies consists of administering a scabicidal agent in combination with an antipruritic agent. An antimicrobial agent may be added if the lesions have become infected with bacteria. Topical scabicidal agents include permethrin 5%, lindane, malathion, sulfur in petrolatum (usually 6%), crotamiton, and benzyl benzoate. Of these agents, permethrin is the most commonly used in the United States, and it is recommended as a first-line therapy by the Centers for Disease Control and Prevention (CDC). Permethrin is applied from chin to toes and for a period of 10-12 hours, after which it should be washed off. The treatment is repeated in 1 week. Hypersensitivity reactions to permethrin have been documented, and there seems to be minimal systemic toxicity caused by minimal skin absorption. Permethrin is the recommended drug of choice for infants (age >2 mo), children, and pregnant (Class B) and nursing mothers. Antihistamines and topical antibiotics may be used as well to decrease pruritus and treat any skin infection. Ivermectin, an oral scabicide, is used in the United States for refractory patients or patients who cannot tolerate topical treatments (although it is not yet approved by the US Food and Drug Administration [FDA]). Preventing scabies reinfestation in family members and close contacts to the affected person is paramount; therefore, all household members should be treated regardless of their symptoms (or lack thereof). In addition, all clothing and linen that may have been in contact with the affected person should be washed in hot water and machine-dried. This process should be repeated 1 week later. Carpets and upholstered furniture should be vacuumed, and the vacuum bags should be disposed of. Patients should be reexamined 2 weeks after treatment to ascertain the treatment effectiveness.
In this patient, treatment with topical permethrin 5% cured the infestation; however, he developed an allergic reaction to the permethrin cream that manifested as widespread pruritic, papular, 1-mm lesions on his knees and elbows. His condition responded to a 1-week course of oral antihistamines (diphenhydramine hydrochloride) and topical zinc oxide lotion. Although they were asymptomatic, all household contacts were treated along with the family members who had been on vacation with the patient.
9
Painful Left Knee in a 7-Year-Old Boy
Background
A 7-year-old boy presents to the emergency department (ED) complaining of left hip and knee pain and refusing to bear weight on his left leg. His mother reports that the child fell down in the street 2 days ago. The pain is described as mild to moderate in intensity. The patient denies any numbness or tingling in the distal left lower extremity. No skin laceration or bruising is reported by the family. Upon further questioning, the mother admits to having witnessed evolving symptoms over the past 2 months. She describes a worsening limp, decreasing mobility, and increasing left hip and knee pain, without any identified inciting trauma.
The patient was brought to the ED 10 months before this presentation for a similar episode of left knee pain after a vague history of a fall. At that visit, radiography of the affected knee demonstrated changes consistent with a mild form of unilateral left-sided juvenile Blount disease. The patient was given a prescription for a nonsteroidal anti-inflammatory medication as needed for pain control, and he was discharged without hospital admission. The symptoms resolved after only 3 days, and the child was asymptomatic for the following 8 months. The past medical history is remarkable for the use of an albuterol inhaler to treat intermittent episodes of asthma. The patient is also markedly obese. He has no previous history of surgery and no known drug allergies. The patient is up to date on his immunizations. The patient's mother denies any exposure of the child to cigarette smoking. The family history is noncontributory.
On physical examination, the patient has a temperature of 98.2°F (36.8°C). He has a pulse of 82 bpm and a blood pressure of 138/74 mm Hg. The patient's respiratory rate is 19 breaths/min, and his oxygen saturation is 99% while he is breathing room air. The patient is obese in appearance. His weight is 181 lb (82 kg), well above the 95th percentile for boys of the same age. He is alert, oriented × 3, and in no acute distress. The neurovascular examination reveals normal pulses, capillary refill, sensation, and motor function. The right and left upper extremities are unremarkable. The right lower extremity is normal. The left hip has a decreased range of motion, with pain elicited on passive internal rotation while the patient is lying in the supine position and positive tenderness to palpation at the left groin. The left knee is tender to palpation and has a decreased range of motion, with no swelling, laxity, or effusion. The left ankle and foot examinations are unremarkable. The patient refuses to bear weight on the left lower extremity and demonstrates an antalgic gait. The skin has no abrasions, ecchymosis, or lacerations.
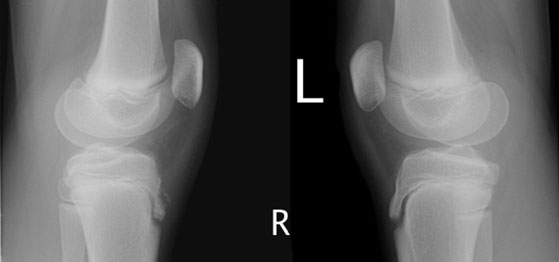
A frontal radiograph of the knees (image not provided) shows abnormal medial metaphyseal beaking of the left proximal tibia and mild flattening of the epiphysis medially. The right knee is normal. When compared with the previous radiographs of the knees obtained 10 months earlier, no significant change is noted for the patient's mild form of unilateral left-sided juvenile Blount disease. A review of the frontal and lateral radiographs of the hips obtained 10 months ago (images not provided) demonstrates that the left hip showed only slight physeal widening compared with the right hip, but it was otherwise unremarkable. Films of the hips at the present visit (Figures 1 and 2) show an interval change.
The peak age for this diagnosis is between 4 and 8 years.
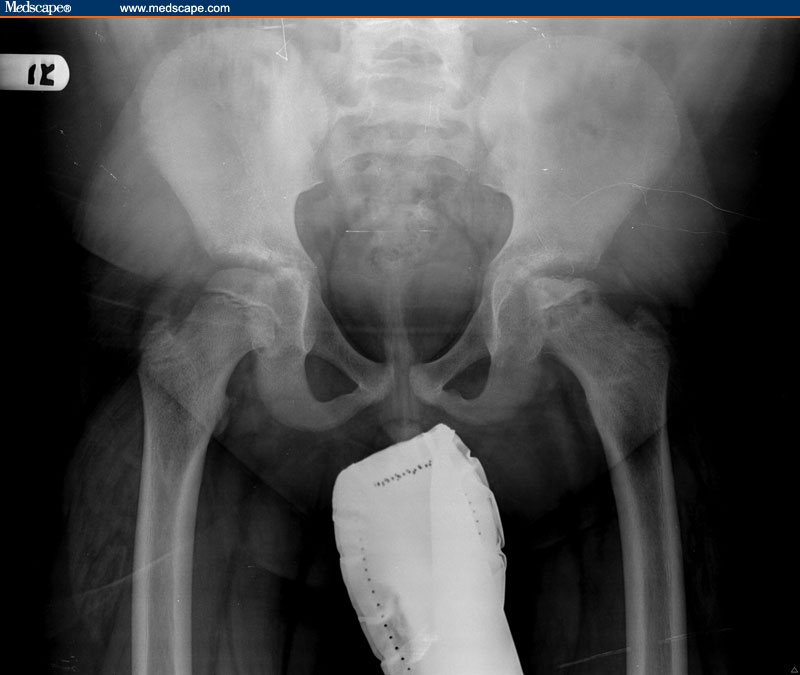
The frontal radiograph of the hips (Figure 1) shows loss of the normal spherical shape of the left capital femoral epiphysis in comparison with the contralateral lower extremity. The contour is flattened; the flattening is most severe along the lateral aspect. The left femoral ossification center is sclerotic, small in size, and presents with slight subluxation laterally. Radiolucent fissures are present superiorly. A large irregular cystic lucency occupies the left proximal femoral metaphysis. The frog-leg radiograph of the hips (Figure 2) demonstrates prominent widening of the physis on the left.
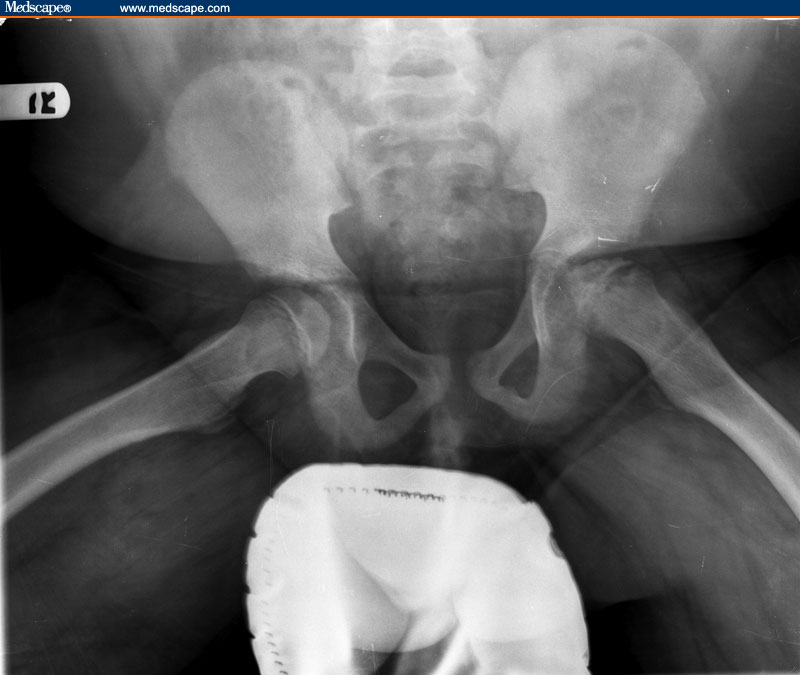
Legg-Calvé-Perthes disease is a pediatric disorder that afflicts children between the ages of 3 and 12 years. The peak incidence for this condition occurs between the ages of 4 and 8 years. Males are affected more frequently than females, by about a 5:1 ratio. Legg-Calvé-Perthes disease occurs most often in white children. About 90% of cases are unilateral in presentation. The remaining 10% of cases often present with apparent unilateral symptoms, only to develop disease of the contralateral hip at a later date. Involvement of both hips at the same time is uncommon, and this sequence of bilateral hip findings can help differentiate Legg-Calvé-Perthes disease from other disorders.
Osteonecrosis of the capital femoral epiphysis is the eventual outcome of this disease process. A vascular insult to the blood supply of the epiphysis leads to infarction of the trabecular bone, flattening, and structural collapse of the proximal femur, with associated overgrowth of the articular cartilage. The cause of the ensuing vascular compromise is not well understood.
Several hypotheses relating to the etiology of Legg-Calvé-Perthes disease have been postulated; these include exposure to passive smoking, low socioeconomic status, trauma, genetic factors, and dietary deficiencies. Despite extensive research, however, no proven cause has been described, and the disease remains idiopathic in nature.
Generally, Legg-Calvé-Perthes disease progresses in 3 successive phases: a period of destruction, followed by a stable phase and, lastly, a healing phase. Each phase may occur over a period of months to years. The chondral surface of the hip joint is not usually directly involved, but it may be secondarily affected at later stages by joint incongruity. The magnitude of the abnormal structural changes to the proximal femur is variable, and it depends on the extent of osteonecrosis and the amount of mechanical pressure exerted on the joint.
Dead bone is reabsorbed, and revascularization and restoration of the epiphysis occur. The shape of the femoral head will reflect the severity of the original vascular insult and the influence of treatment. The femoral head can be restored to a normal appearance similar to that of the unaffected contralateral hip, or it can become flattened (coxa plana) and widened (coxa magna), with the development of coxa vara. As a result of these changes, leg-length discrepancy and degenerative joint disease can develop.
Imaging plays an important role in establishing the diagnosis of Legg-Calvé-Perthes disease, grading the disease severity, and guiding clinical treatment. Early radiographic signs are nonspecific and related to synovitis. There is capsular bulging, with displacement of the fat pads around the capsule; more characteristic features include the appearance of a subchondral peripheral fracture of the femoral epiphysis (crescent sign). This fracture is best seen when the patient is in the frog-leg position. Later stages show a decrease in size, sclerosis, flattening, fragmentation, and fissuring of the epiphysis. These findings represent a combination of processes, including compaction and infarction of bone trabeculae, new bone formation, calcification of saponified fat in the marrow, and relative density to the adjacent osteopenia. The epiphysis may also become displaced laterally, producing the appearance of medial joint space widening. A lucent and cystic metaphysis is an accompanying sign. Widening of the femoral head and neck (coxa magna) can occur. The greater trochanter appears larger when compared with the shortened femoral neck. Coxa plana and coxa vara may be radiographic findings seen in chronic disease. Magnetic resonance imaging (MRI) is useful for early diagnosis when radiographs are normal or when the diagnosis is in doubt. MRI can demonstrate areas of low signal intensity within the capital femoral epiphysis on T1- and T2-weighted images, which is consistent with early osteonecrosis. Revascularization is demonstrated as a bright signal, similar to fat on T1-weighted images. The asterisk sign (low signal intensity on T1-weighted images and bright signal intensity on T2-weighted images in the epiphyseal marrow) and the double-line sign (a low signal intensity line representing the line between the necrotic and viable bone and a bright signal intensity line representing the granulation tissue) are seen in 80% of cases. Articular cartilage irregularities and joint effusion have bright signal intensity on T2-weighted images. MRI also provides excellent prognostic information by revealing the extent of infarction, growth plate abnormality, and loss of femoral containment within the acetabulum. Bone scintigraphy, arthrography, and ultrasonography can serve as adjunctive modalities in some cases.
The differential diagnosis for Legg-Calvé-Perthes disease includes focal and systemic disorders that can cause osteonecrosis of the hip. Systemic diseases that can lead to osteonecrosis of the hip include sickle cell anemia and other hemoglobinopathies, Gaucher disease, steroid arthropathy, Cushing syndrome, and hypothyroidism; focal processes that do so include posttraumatic osteonecrosis, posttreatment osteonecrosis in congenital hip dislocation, slipped capital femoral epiphysis, and inflammatory arthropathies. Inflammatory diseases can induce osteonecrosis of the hip as well; these include toxic synovitis, septic arthritis, osteomyelitis, synovitis secondary to osteoid osteoma, and juvenile chronic arthritis. Other hip dysplasias include multiple epiphyseal dysplasia and, in children younger than age 5 years, Meyer dysplasia (which is localized to the epiphysis only).
Children with Legg-Calvé-Perthes disease most commonly present with a limp as well as hip and/or knee pain. The family often denies an incident of associated trauma, although an inciting injury may be reported in a minority of cases. Some children without pain are unexpectedly diagnosed with the disease. On physical examination, an antalgic gait, thigh atrophy, and decreased range of motion to internal rotation and abduction of the hip are found. Pain is also elicited on palpation of the groin.
Children between the ages of 3 and 5 years generally have the best prognosis for Legg-Calvé-Perthes disease, whereas those over the age of 8 years typically have the worst prognosis. Several classification schemes have been devised to grade the severity of disease based on radiographic findings, with no consensus as to the best classification system. The Catterall criteria are one example of a well-established and commonly used grading system. Group I involves less than one fourth of the epiphysis, affecting only the anterior portion. No crescent sign, collapse, sequestration, or metaphyseal involvement is seen. Group II shows more advanced changes affecting the anterior portion, but not more than one half of the epiphysis is affected. Osseous structural abnormalities, the crescent sign, and collapse are seen, with possible focal abnormalities within the metaphysis. Group III encompasses nearly the entire epiphysis, with diffuse metaphyseal abnormalities. Group IV represents the complete involvement of the epiphysis. Children in Groups III and IV have the worst prognoses; however, those patients in Groups I and II may develop more advanced disease at follow-up. Additional Catterall risk factors for potential increased morbidity include a "V" shaped radiolucency in the lateral aspect of the epiphysis (Gage sign), lateral subluxation of the epiphysis, calcification lateral to the epiphysis, and a horizontal appearance of the physis.
Important priorities in the treatment of Legg-Calvé-Perthes disease include maintaining the spherical shape of the femoral head and containing its presence within the acetabulum. Alleviation of symptoms and restoration of range of motion of the lower extremity are desired clinical outcomes. A range of therapeutic strategies are employed for patient management; however, controversy still exists over which methods of intervention are optimal for each patient and how to best grade the severity of disease. The general philosophy of the surgeon on these matters typically dictates the treatment plan. Management is directed by the patient's symptoms and the severity of the disease, as determined by imaging studies. The bone age of the child is another important consideration for treatment. Patients younger than the age of 6 years or older children whose condition is classified in Catterall Group I may require only conservative management, without surgery. These children may receive range-of-motion exercises, traction, avoidance of weight bearing, and/or bracing as the only therapy. In certain children, only bed rest and anti-inflammatory medications may be indicated. In older children or in those with advanced disease (Catterall Groups III and IV), surgical intervention may be necessary. Various procedures are employed, depending on the clinical situation and surgeon's preference, to avoid long-term disability.
The patient in our case was placed on crutches and given instructions not to bear weight on the left lower extremity. Following discharge from the ED, he received follow-up evaluation by the pediatric orthopedics service of an affiliated institution, and he subsequently received surgical management 4 months later.
10
A Puzzling Facial Rash on a 17-Year-Old Boy
Background
A 17-year-old male high school student presents to the pediatric infectious disease clinic complaining of a 10-day history of a facial rash that "won't get better." The patient had previously visited his primary care provider (PCP), who started the patient on amoxicillin-clavulanic acid 8 days ago. The rash did not improve on the antibiotic, and as a result, it was discontinued and the patient switched to trimethoprim-sulfamethoxazole. No improvement was noted with the second round of antibiotic therapy; the rash continued to spread, and the lesions increased in number. The patient was subsequently advised to follow up with the infectious disease clinic.
At the infectious disease clinic, the patient states that the rash started with several pimples over the forehead and cheek and then continued to spread and involve most of the right side of his face. The lesions are not itchy, but they are painful. The patient has no known drug allergies. His immunizations are up to date. He is very active on the wrestling team and was happily preparing for an upcoming competition. The patient denies having any weight loss, headaches, dizziness, photophobia, fever, or chills. The family history is non-contributory.
On physical examination, the patient is alert and orientated. His oral temperature is 97.0°F (36.1°C). The patient has normal heart sounds, his pulse has a regular rhythm of 97 bpm, and his blood pressure is 125/75 mm Hg. His lungs are clear, and his respiratory rate is 12 breaths/min. The examination of the head, eyes, ears, and nose is remarkable for multiple vesicular lesions measuring about 0.5 cm in diameter (see Figures 1 and 2). There is bilateral submandibular lymph gland enlargement measuring 1.5 cm by 1 cm. The neck is supple. His abdomen is soft and nontender to deep palpation in the epigastric region, and no organomegaly is noted. A complete blood count (CBC) taken at the PCP's office showed a white blood cell (WBC) count of 7.4 × 103/µL (7.4 × 109/L), with a normal differential; a hemoglobin of 13.6 g/dl (136 g/L); a hematocrit of 38.3% (0.3830); and a platelet count of 298 × 103/µL (298 × 109/L).
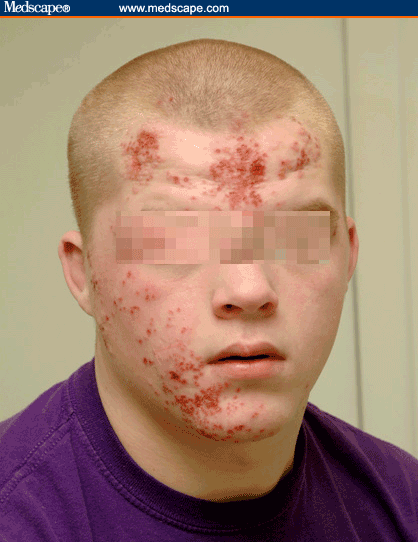

Hint: The patient was disqualified from wrestling because of his lesions.
Herpes gladiatorum (HG) is an infection caused by the herpes simplex virus (HSV) type 1. HG most commonly occurs among wrestlers and other athletes who participate in close skin contact sports such as wrestling (herpes gladiatorum) and rugby (herpes rugbiaforum). HG is also known as "mat herpes" among wrestlers. HG is spread by direct skin-to-skin contact. The lesions appear within 7 to 14 days after exposure; however, in some cases the lesions take longer to appear. Our patient presented with a primary herpes gladiatorum (PHG) infection, which is usually more severe than the recurrent infections. His lesions presented with disseminated vesicles, punched-out erosions, and central crusting on the forehead and right cheek. The patient was at an increased risk of contracting PHG because of his participation in wrestling. Herpes simplex virus DNA was detected with a polymerase chain reaction (PCR) examination.
Outbreaks of HG have occurred countless times during the last decade; several outbreaks of HG have occurred among wrestlers across the United States. It is critical that health care providers, athletic trainers, coaches, and athletes recognize the threat of HG skin infection to minimize the risk of outbreaks. Vigilant surveillance and appropriate antiviral treatment will curtail the transmission of HG among wrestlers.Lack of proper understanding of PHG disease could lead to misdiagnosis and frequent outbreaks.
HSV is a double-stranded DNA virus. About 80% of adults have antibodies to HSV-1, and about 20% of the population has antibodies to HSV-2. HSV-1 is commonly known to cause herpes labialis and keratitis. Most childhood HSV infections are caused by HSV-1. HSV-2 is commonly known to cause genital herpes infections, which is one of the most common sexually transmitted diseases in the United States. HSV-2 is transmitted primarily by direct contact with lesions, and it is most often transmitted venereally. Generalized or localized cutaneous and mucosal lesions characterize initial infection by HSV. Recurrent infections are milder because fewer viruses are shed and a stronger immune response is elicited. HSV remains dormant in the nerve ganglia; febrile illness, stress, immunosuppressive drugs, and ultraviolet light can precipitate recurrent eruptions. In rare cases, the initial replication of HSV can lead to meningitis or encephalitis. HSV persists for life in a latent form. The virus is sometimes confused with herpes zoster because the site of latency for HSV is the trigeminal ganglion. HSV may also manifest as a severe and/or life-threatening infection in immunocompromised individuals and in newborn babies. Specifically, disseminated infections can result in esophagitis, pneumonitis, encephalitis, hepatitis, and adrenal necrosis.
Currently, no epidemiologic studies accurately identify the true incidence of HG; however, the National Collegiate Athletic Association (NCAA) estimated an incidence of HG as high as 40% among wrestlers.The most common locations of HG, in descending order, are the head, face, neck, chest, and shoulders. Typically, lesions will be observed on the head, face, neck, cheeks, forehead, shoulders, and arms. According to most studies, about two thirds of wrestlers will have the herpetic lesions on the right side of the body. Hence, HG is transmitted during close skin-to-skin physical contact, known in wrestlers' terminology as the "lock-up position."
PHG generally presents with an erythematous rash, sore throat, fever, cervical lymphadenopathy, and vesicles. Occasionally, the HG lesion will lack the grouped vesicles on an erythematous base, and it is sometimes mistaken for impetigo, acne, tinea corporis, atopic dermatitis, varicella, or scabies. A significant complication of PHG in wrestlers is dendritic keratitis with subsequent corneal scarring. Other ocular complications of PHG include conjunctivitis, scleritis, and uveitis. Fluid from the base of unroofed vesicles can be sent for testing by PCR, the test of choice for diagnosing PHG. A Tzanck smear of scrapings from the base of vesicles will demonstrate multinucleated giant cells, a finding that is highly indicative for HSV infection.
The NCAA has developed recommendations on "time until return to competition" for primary herpes gladiatorum and for recurrent infection. For a primary outbreak of herpes, the wrestler must be examined by a clinician or an experienced certified athletic trainer; the following recommendations must be met before a wrestler returns to competition:
- The athlete must have no signs of systemic symptoms of viral infection.
- The athlete must be free of any new lesions for 3 days or more prior to start of competition.
- The skin lesions must be dried and surmounted by a firm adherent crust.
- The athlete must have been on appropriate antiviral therapy for at least 120 hours before the beginning of a competition.
For recurrent HG, the NCAA has also established several recommendations. First, vesicles must be completely dry and crusted. Second, the wrestler must have been on the appropriate dosage of antiviral therapy for 120 hours or more at the time of tournament. For questionable cases, a Tzanck preparation should be performed, and the wrestler's status should be deferred until Tzanck prep or HSV assay results are available.
Antiviral drugs with activity against viral DNA synthesis have been effective against PHG infections. Acyclovir, famciclovir, and valacyclovir inhibit virus replication and suppress clinical manifestations, but they are not a cure for PHG, since HSV remains latent in sensory ganglia. Oral acyclovir has been shown to be effective in suppressing PHG in wrestlers; it is the drug of choice for treating PHG. Acyclovir reduces the duration of symptomatic lesions and is indicated for patients presenting within 2-3 days of the appearance of a herpetic rash. Most patients on acyclovir will experience less pain and quicker resolution of their vesicular lesions. Several effective treatments for adult patients include oral acyclovir 200 mg 5 times daily or 400 mg 3 times daily for 7-10 days or until clinical resolution occurs. The recommended dose of acyclovir for PHG in the pediatric age group is 20-30 mg/kg/d, in 5 divided doses, for 7-10 days. As with all infections, prevention is better than treatment.
In addition to the above treatment, wrestlers must practice effective hygiene immediately after wrestling. It is critical to frequently clean competition gear and change towels. Regular hand-washing and thorough cleaning of the mats are critical. Wrestling mats should be cleaned between matches with household bleach (one-quarter cup of bleach in 1 gallon of water). Early identification and treatment can allow the wrestler to return to participation earlier and prevent teammates from contracting the disease. Despite these precautions, PHG will spread during wrestling and other close-contact sports resulting from contact with asymptomatic infected athletes.
The patient in this case was started on acyclovir 400 mg 3 times a day for 1 week at the first visit to the pediatric infectious disease clinic. It was strongly suspected that his lesions were caused by PHG, since there had been no improvement with the 2 courses of antibiotic treatment that had been initially tried by his primary care provider. After 1 week of acyclovir, most of his facial lesions were dry and had an adherent crust; however, there were still 2 moist lesions on his right shoulder, which resolved completely by the second week of treatment. The patient and his parents were advised repeatedly to call and report any eye symptoms, seizure, alteration of mental status, personality changes, photophobia, or headaches. It was noted on subsequent follow-up that the patient's 13-year-old brother had developed similar lesions; it was later learned that he had borrowed his older brother's headgear, which was the most likely the cause of his lesions. The rash on the brother also resolved after starting acyclovir.